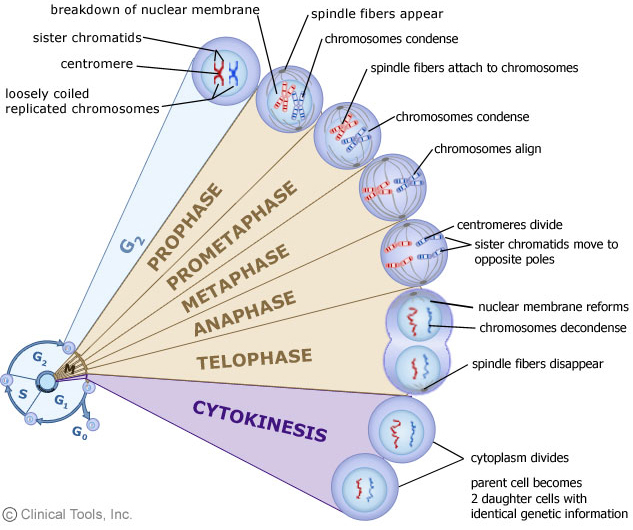
• Mitosis: 有丝分裂; Meiosis: 减数分裂
Interphase: 分裂间期; Prophase: 分裂前期; Metaphase: 分裂中期; Anaphase: 分裂后期; Telophase: 分裂末期
• Histone: 组蛋白 hhistidine 组氨酸
• Nucleosome: 核小体
•Chromosome: 染色体; Chromatin: 染色质; eu- 真染色质; hetero- 异染色质
===================================================The total length of DNA in a eukaryotic cell depends on the species, but it can be thousands of times as much as in a prokaryotic genome.
Eukaryotic chromosome is made up of a number of discrete bodies called chromosomes. The DNA in each chromosome is believed to be a single linear molecule, which can be up to several centimeters long.
All these each contain a long linear DNA molecules, which must be packaged into the nucleus, a space of approximately the same volume as a bacterial cell
SO, much longer DNA chains packaged into a space of the same volume as a bacterial cell? → for example, 2 cm of DNA length versus ~10 µm of cell size for fruit fly; most condensed form of human chromosome is about ~2 µm long = 10,000× packing ratio
the obvious result is in their most highly condensed forms, the chromosomes have an enormously high DNA concentration: perhaps 200 mg/ml.!
The feat of packing is accomplished by the formation of a highly organized complex of DNA and protein, known as the chromatin, a nucleoprotein complex. (←our hero today, has finally showed up.)
Chromosomes greatly alter their level of compectness as cells progress through the cell cycle, vary between highly condensed chromosomes at metaphase(just before the cell division), and very much more diffuse structures in interphase.(This implies the existence of different levels of organization of chromatin)
More than 50% of the mass of chromatin is protein. Most of the protein in eukaryotic chromatin consists of histones, of which there are five families: H2A, H2B, H3 and H4, known as the core histones, and H1.
The core histones are small proteins, with masses between 10 and 20 kDa, and H1 histones are a little larger at around 23 kDa.
The unified atomic mass unit (symbol: u) or dalton (symbol: Da) is the standard unit that is used for indicating mass on an atomic or molecular scale (atomic mass). One dalton is approximately the mass of a nucleon and is equivalent to 1 g/mol.[1] It is defined as one twelfth of the mass of an unbound neutral atom of carbon-12 in its nuclear and electronic ground state,[2] and has a value of 1.660538921(73)×10−27 kg.[3]
All histones proteins a large positive charge; between 20 and 30% of their sequences consist of the basic amino acids, lysine and arginine. This means that histones will bind very strongly to the negatively charged DNA in forming chromation.
amino acids in English?
Members of the same histone class(family) are very highly conserved between relatively unrelated species, for example between plants and animals, which testifies to their crucial role in the chromation.
Within a given species, there are normally a number of closely similar variants of a particular class, which may be expressed in different tissues, and at different stages in development.
There is not much similarity in sequence between the different histone classes, but structural studies have shown that the classes so share a similar tertiary structure, suggesting that all hisotnes are ultimately evolutionarily related.
H1 histones are somewhat distinct from the other histone classes in a number of ways; in addition to their larger size, there is more variation in H1 sequences both between and within species than in other classes. Histone H1 is more easily extracted from bulk chromatin, and seems to be present in roughly half the quantity of the other classes, of which there are very similar amounts.
Next section we will cover the distinct role of histone Hi in chromatin structure.
![the blueprint of life [8]: eukaryotic structure of DNA 1(chromatin)](https://apbiology.cn/wp-content/uploads/sites/8/2014/01/m-bio-8-chromotin.png)
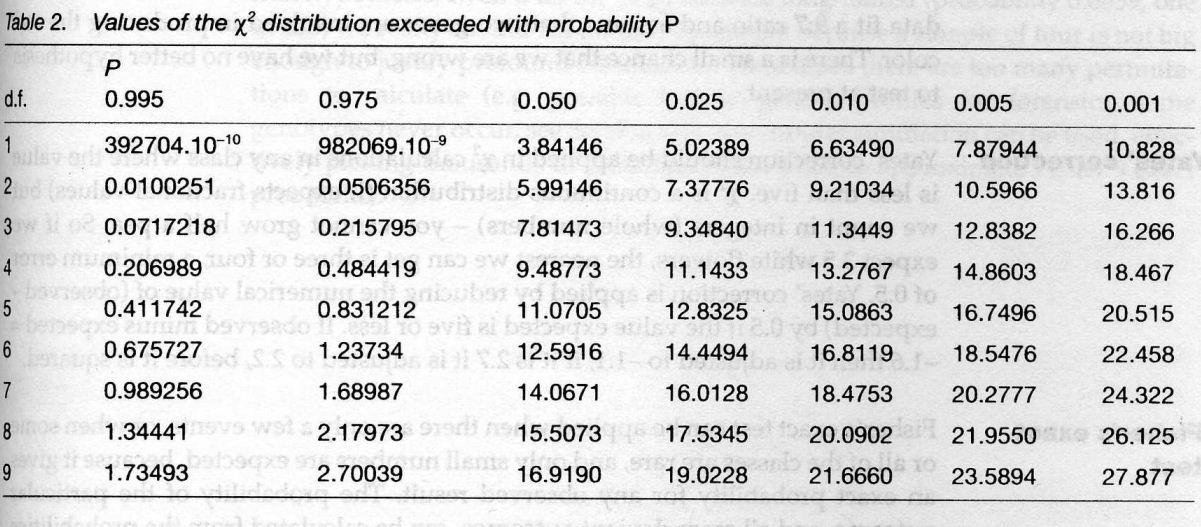
![Mendel’s Genetics [7]: handling problems](https://apbiology.cn/wp-content/uploads/sites/8/2013/12/chi-square.jpg)
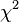 test)=
sum[(obseved expected)ˆ2/expected]
test)=
sum[(obseved expected)ˆ2/expected]
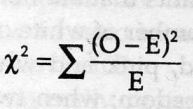
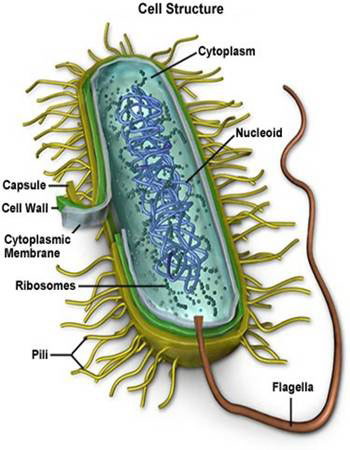
![the blueprint of life [7]: prokaryotic chromosome structure of DNA](https://apbiology.cn/wp-content/uploads/sites/8/2013/07/m-bio-7-bacteria.jpg)

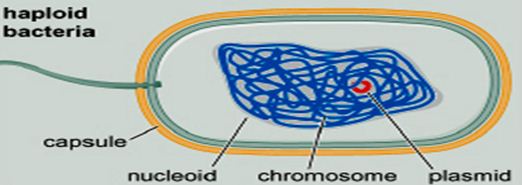 ——————————————————————————-
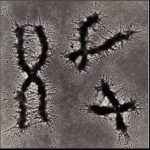
——————————————————————————- Remember this famous electron micrograph of an E. coli cell we showed before?
Remember this famous electron micrograph of an E. coli cell we showed before? 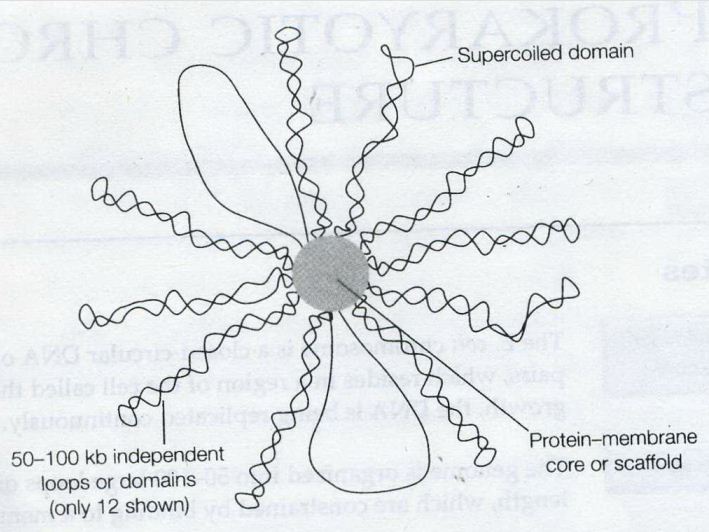

























![the blueprint of life [6]: Chromosomal Structure of DNA 1](https://apbiology.cn/wp-content/uploads/sites/8/2013/12/m-bio-6-chromosomes.jpg)














![Mendel’s Genetics [6]: Examples of epistasis](https://apbiology.cn/wp-content/uploads/sites/8/2013/12/genetics-3-snapdragons.jpg)
![The blueprint of life [5]: spectroscopic and thermal properties of DNA](https://apbiology.cn/wp-content/uploads/sites/8/2013/07/UV-absorption-of-DNA.jpg)
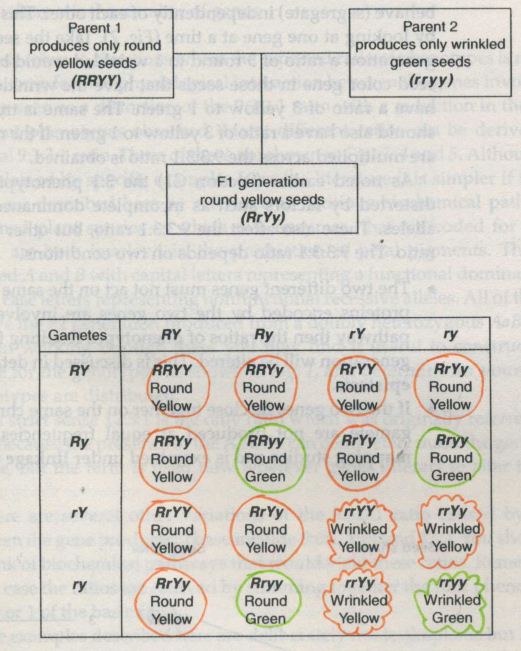
![Mendel’s Genetics [5]: The dihybrid cross](https://apbiology.cn/wp-content/uploads/sites/8/2013/07/genetics2.png)